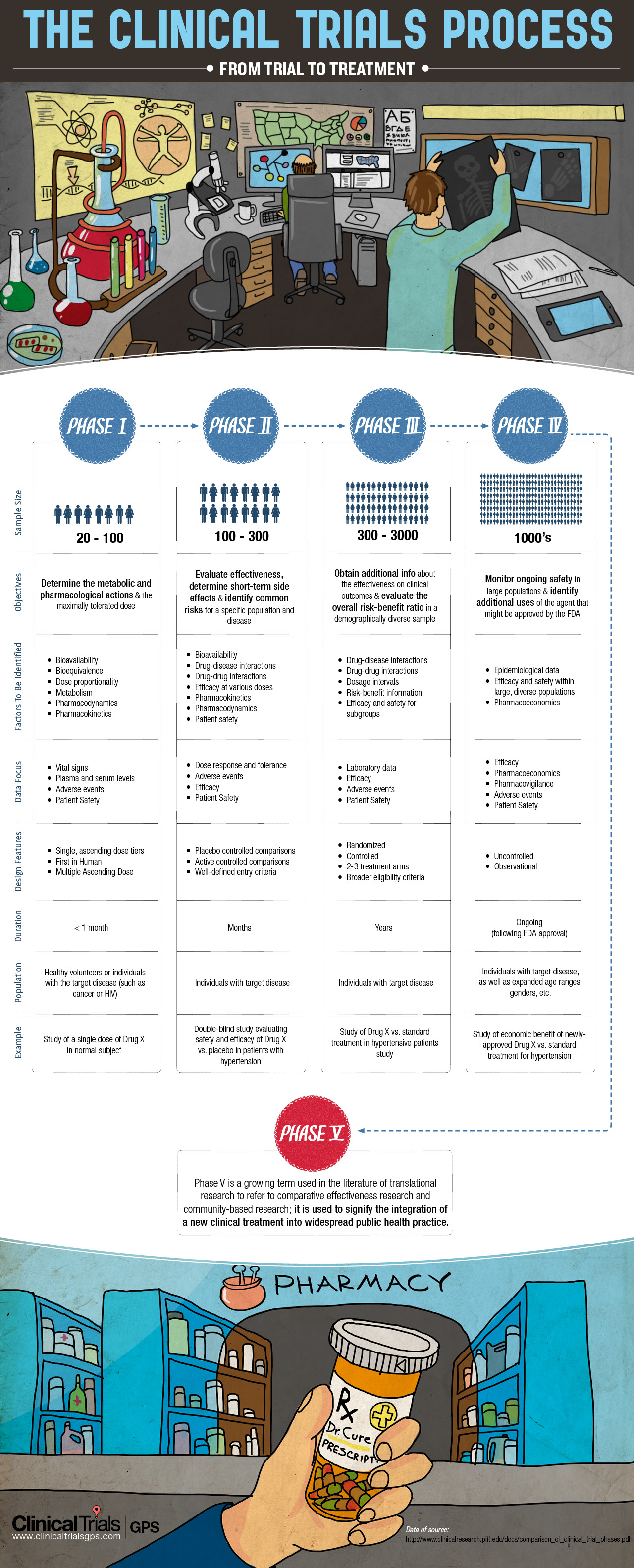
Understanding the Phases of Clinical Trials (INFOGRAPHIC)
The entire process of a clinical trial is broken up into four phases, each of which serves the research team a unique purpose in order to better study the products and achieve the protocols.
click here to view larger image
Phase 0 Clinical Trials
Phase 0 of clinical trials consists of 10-15 participants. This is the first time that the drug or treatment is being evaluated in humans to determine its metabolic and pharmacological effectiveness when taken while fasting as opposed to after being fed, its tolerability, side effects and overall safety. Drugs that make it through this phase go on to begin the four phases of clinical trials.
Phase I Clinical Trials
Phase I trials consist of about 20-100 participants that test a drug over the course of a few weeks to determine its metabolic and pharmacological effects. These trials are normally conducted in a clinical trial clinic so that the participants can be watched carefully at all times. Phase I may consist of dosage escalation studies so that the researchers can determine the safest dose to administer. In single ascending dose studies, small groups of participants are given a single dose of the drug being studied, and when rendered safe for the dosage to be escalated, different groups of people are given the higher dosages until the maximum tolerated dose (MTD) is attained. In multiple ascending dose studies, a group of patients is given multiple low doses of the drug while having their urine and blood are analyzed at various times, and when the dose is rendered safe for escalation, new groups of participants are given the higher doses up until the MTD is reached.
Phase II Clinical Trials
In Phase II, groups of 100-300 test the studied drug to further evaluate its efficacy, short-term side effects and potential common hazards for a specific population and disease upon the treatment or drug’s confirmed safety in phase I. Most of the determined failings discovered about experimental drugs are discovered during phase II, if any are ever found at all. Phase II continues to study the efficacy and required dosage. Phase III, the most well-known of all the phases, are randomized, controlled, large-scale studies that involve 300-3,000 or more participants. These multicenter studies compare the experimental drug to the current FDA-approved treatments.
Phase III Clinical Trials
Phase III is the most costly and tedious of all the phases, and it lasts for years. At this phase, researches observe whether the drug could be used to treat other diseases beyond their original intent. Phase III’s focuses are to obtain additional data about the drug’s effectiveness on clinical outcomes and to analyze the ratio of benefits to risks of the drug or treatment in a demographically diverse population of study participants. To allow market sales, the FDA typically requires two positive cycles of phase III trials.
Phase IV Clinical Trials
In phase IV, ongoing technical support and safety surveillance are performed. The patient population consists of thousands of participants. Phase IV trials are ongoing, following the approval of the FDA.
Phase V Clinical Trials
The term “fifth phase” has been contemporarily coined and used in the literature of translational research. This phase consists of the post phase IV integration of a new drug into widespread public use. In this phase, community-based research is compared to the clinical comparative effectiveness research of this new drug or treatment.