Understanding the Process of Clinical Research Trials
Before participating in a clinical trial, participants must be recruited and screened for their health. Such health examinations may include being measured for height, being weighed, having blood pressure recorded, having an ecocardiogram and having a blood test and urinalysis. Other examinations may be necessary for the trial depending upon what the medical staff deems necessary in order to properly test the drug or treatment therapy.
Upon acceptance to join the trial by the affiliated medical researchers, participants sign legal document called “informed consent”. This document states that the study subjects have been briefed by the medical staff involved in the study on the possible risks, duration and details of the protocols. Trial subjects have the option of withdrawing from the study without penalty even after they have signed the document.
Every clinical trial follows an assigned protocol, or detailed plan. The protocol describes the schedule of tests and procedures, the duration of the entire study, the qualifications that participants must meet in order to join the study and the drug/treatment to be tested. The medical team closely follows the protocol and carefully monitors the participnts to ensure that the protocol is properly being followed. The health of the particiapnts is also monitored in order to protect the participants and to accurately determine the safety and efficacy of the drug or treatment being tested.
Many clinical trials use a placebo in order to more accurately determine the efficacy of the experimental drug or treatment. A placebo is a false drug that offers no remedial value to the trial (or in general). Studies that use this observational method give the placebo to a control group while the real experimental drug or treatment is given to a different group of patients. In a single blind study, the participants do not know which group they are part of, and in some cases neither does the medical staff, making the trial a double blind study.
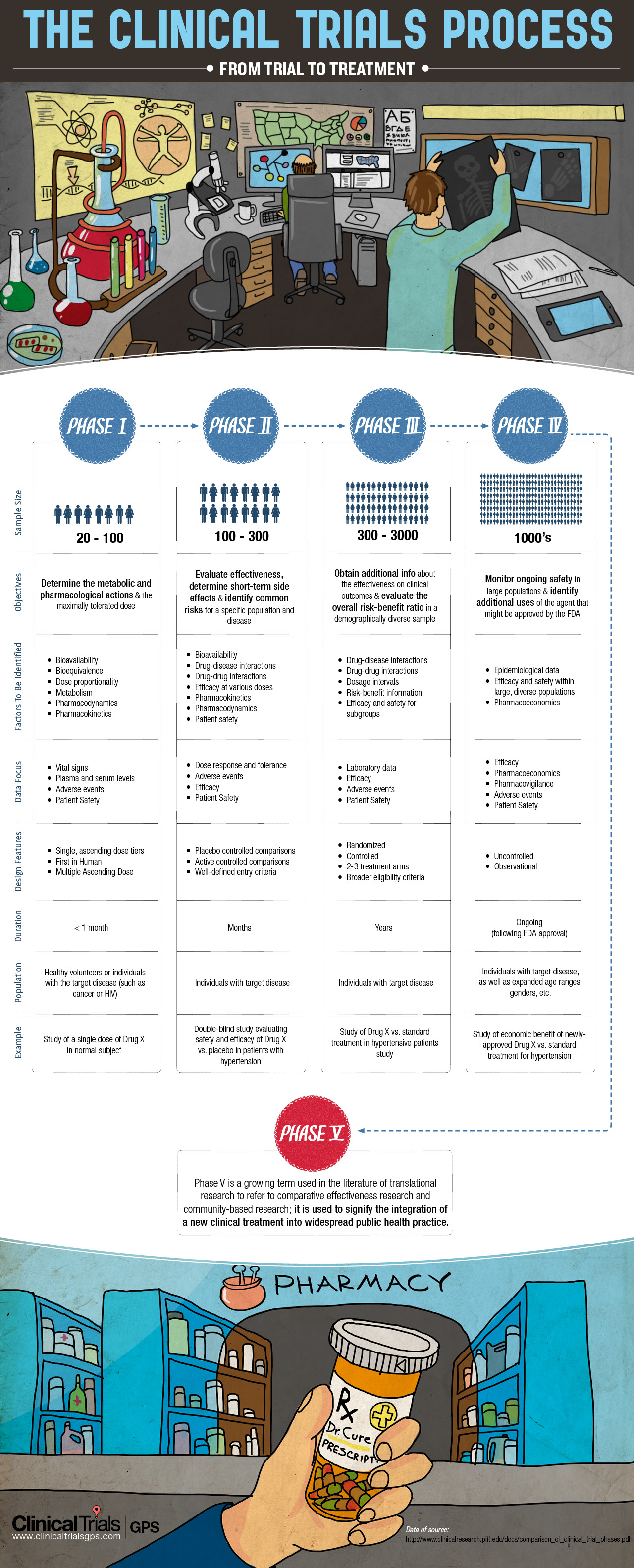
The entire process of a clinical trial is broken up into four phases, each of which serves the research team a unique purpose in order to better study the products and achieve the protocols.
- The first phase of clinical trials consists of about 20-100 participants. This is the first time that the drug or treatment is being evaluated in humans to determine its effectiveness, side effects and overall safety.
- In Phase II Clinical Trials, groups of 100-300 test the studied drug or treatment to further evaluate its efficacy and potential hazards upon its confirmed safety in phase I.
- Phase III Clinical Trials, the most well-known of all the phases, are randomized, controlled, large-scale studies that involve 1-3,000 or more participants. At this phase, researches observe whether the drug could be used to treat other diseases beyond their original intent.
- In Phase IV Clinical Trials, ongoing technical support and safety surveillance are performed on a larger patient population and for a longer time duration. Harmful effects of the drug observed in phase IV can result in the drug being pulled from the market or being restricted to certain uses.